How much CO2 is released during fermentation?
•
How much CO2 is released during fermentation?
You see the bubbling in your fermentation tanks every day. But do you know how much gas that really is? This unmeasured stream could be a valuable resource for your plant, if only you could quantify it.
For every molecule of ethanol produced, one molecule of CO₂ is released. In brewing, this means roughly 1 pound of CO₂ is created for every 1 pound of ethanol. A typical 100-barrel (117 hL) batch can generate over 2,500 pounds (1,130 kg) of CO₂.
I often get asked this question when I first visit a new client. Seeing the bubbles is one thing, but understanding the sheer volume of gas being produced is another. The numbers are surprisingly large and highlight the opportunity for recovery. It is not just a byproduct; it is a significant co-product waiting to be captured. This realization is often the starting point for a very productive conversation about efficiency and self-sufficiency.
How much CO2 is in flue gas?
Your boiler or generator produces exhaust that you vent outside. But this flue gas contains a high concentration of CO₂, representing wasted energy and a potential product going up in smoke.
The concentration of CO₂ in flue gas depends on the fuel. Natural gas combustion typically results in 8-12% CO₂ by volume. Coal combustion is higher at 12-15%. The rest of the gas is mostly nitrogen, water vapor, and oxygen.
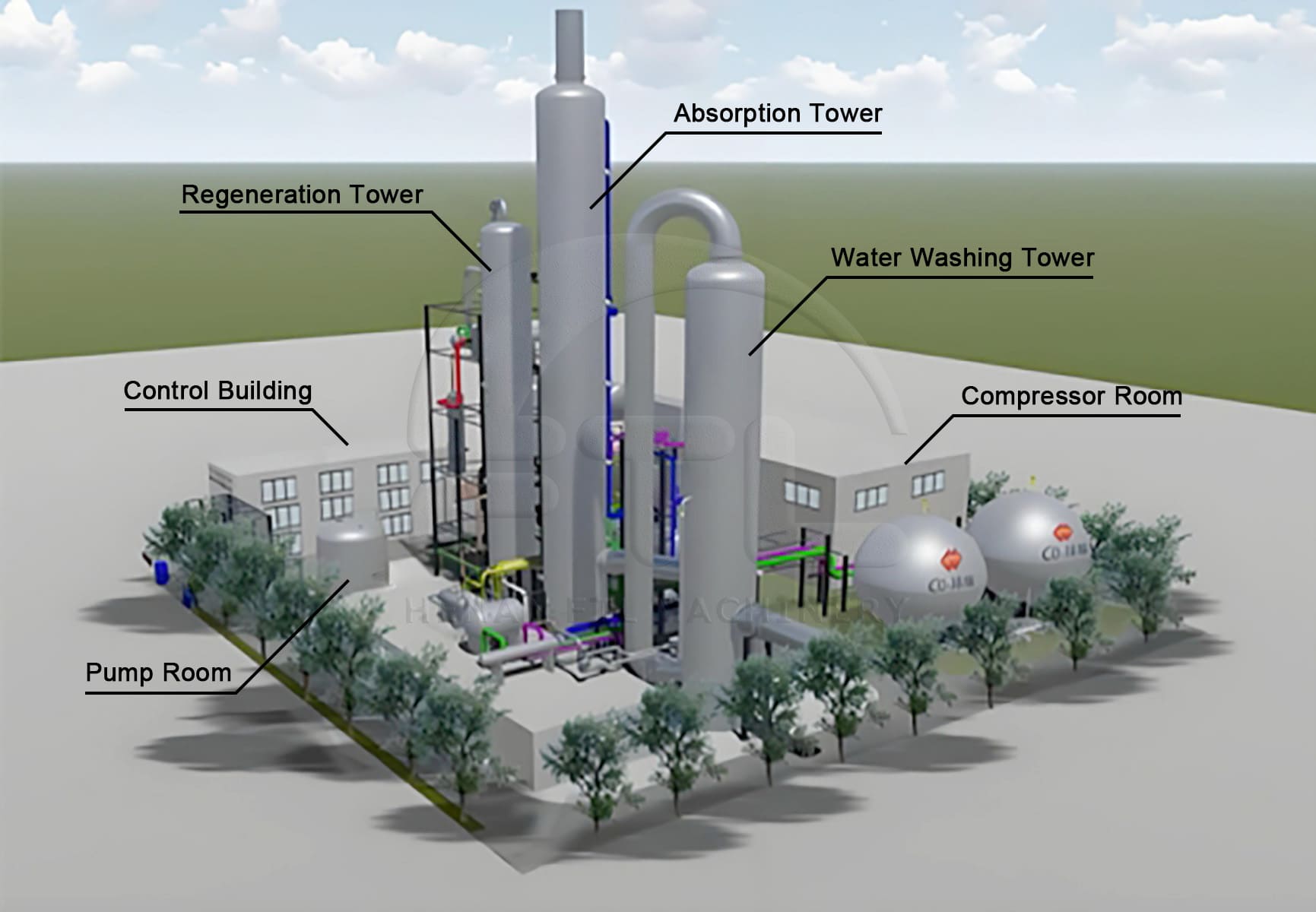
This is a different challenge compared to fermentation CO₂. Fermentation produces a very pure stream, while flue gas is a mixture. This is a crucial distinction for capture technology. I often explain this to clients with combined heat and power (CHP) systems. Capturing CO₂ from flue gas is absolutely possible, but it requires different front-end technology to separate the CO₂ from the nitrogen. The purity of the source gas directly impacts the complexity and cost of the recovery system.
Source Gas Purity Comparison
The starting point matters a lot. A gas that is already highly concentrated is much easier and cheaper to purify into a final liquid product.
| Source | Typical CO₂ Concentration | Key Contaminants | Recovery Complexity |
|---|---|---|---|
| Fermentation | >99% (raw gas) | Water, sulfur compounds, organic volatiles | Low |
| Flue Gas (Gas) | 8% - 12% | Nitrogen (N₂), Oxygen (O₂), NOx, SOx | High |
| Biogas | 35% - 45% | Methane (CH₄), Hydrogen Sulfide (H₂S) | Medium |
As you can see, fermentation gas is the easiest to process because it's already so pure. Flue gas requires a pre-concentration step, adding more equipment and energy use to the project.
How is CO2 removed from flue gas?
You want to capture CO₂ from your boiler exhaust, but it’s mixed with other gases. How do you separate just the CO₂ molecules from this complex stream before you purify it?
CO₂ is primarily removed from flue gas using amine scrubbing. The flue gas is passed through a liquid amine solution that selectively absorbs the CO₂. The CO₂ is later released from the amine by heating it, creating a concentrated stream for further processing.
Amine scrubbing is the leading technology for post-combustion capture. It’s a well-established process used in large-scale industries for decades. From our perspective at FTL Machine, we integrate our CO₂ purification and liquefaction plants after the amine unit. The amine system does the heavy lifting of separating CO₂ from nitrogen, and our system then takes that concentrated CO₂ and refines it into a food-grade liquid. It’s a two-stage process: separation first, then purification.
The Amine Scrubbing Process
This is a continuous loop chemical process. The amines are constantly being recycled to capture more CO₂.
- Absorption: The cool flue gas is bubbled up through a large column called an absorber. A lean amine solution flows down, making contact with the gas. The amines chemically bond with the CO₂ molecules, "pulling" them out of the gas stream. The clean gas, now mostly nitrogen, exits from the top.
- Regeneration: The "rich" amine, which is now loaded with CO₂, is pumped to a second column called a stripper or regenerator. Here, the amine is heated with steam. The heat breaks the chemical bond, releasing the CO₂ as a nearly pure gas.
- Purification: This hot, wet, concentrated CO₂ stream is now the feed gas for a standard CO₂ recovery and liquefaction plant. It is cooled, dried, compressed, and purified to become liquid CO₂. The now "lean" amine is cooled and sent back to the absorber to start the cycle again.
How to capture CO2 from fermentation?
Unlike flue gas, the CO₂ from your fermenters is almost pure. You're wondering what the specific hardware and process steps are to turn this readily available gas into a usable liquid.
To capture fermentation CO₂, a collection line feeds the gas to a booster compressor. It then passes through a purification system to remove water and aromatics before being pressurized and chilled in a liquefaction unit until it becomes a liquid for storage.
This is our bread and butter at FTL Machine. The process is much more direct than flue gas capture because we are starting with a gas that is already over 99% pure CO₂. One of my clients, a mid-sized craft brewer, was amazed at how compact the system was. He expected a massive chemical plant, but what we installed was a self-contained skid that fit neatly in his utility room. We are not performing complex chemical separation; we are simply cleaning up and changing the state of an already very pure gas.
The Fermentation Capture Workflow
The process is a production line designed to polish the gas and make it dense for storage. It is fully automated and designed for reliability.
| Step | Description | Key Equipment |
|---|---|---|
| 1. Collection | Low-pressure, wet CO₂ is collected from the headspace of the active fermentation tanks. | Collection lines, gas balloon buffer |
| 2. Compression | A booster compressor pulls the gas from the balloon and increases its pressure for the purification stages. | Low-pressure CO₂ compressor |
| 3. Purification | The gas passes through filters to remove moisture, sulfur compounds (like H₂S), and volatile organic compounds (VOCs). | Water scrubber, desiccant dryers, carbon filters |
| 4. Liquefaction | The pure, high-pressure gas is chilled by a refrigeration system, causing it to condense into a liquid. | Multi-stage compressor, refrigeration unit |
| 5. Storage | The liquid CO₂ is transferred to a cryogenic, insulated storage tank, ready for reuse. | Insulated storage vessel |
This straightforward mechanical process gives breweries, distilleries, and biofuel plants complete control over their CO₂ supply.
Conclusion
Fermentation releases about one pound of CO₂ for every pound of ethanol. This pure CO₂ is easily captured, while CO₂ from flue gas requires more complex amine scrubbing before purification.
You may also be interested in:

Why is CO₂ recovery technology gaining popularity worldwide?
Why is CO₂ recovery technology gaining popularity worldwide? You see headlines about carbon capture everywhere. But you wonder if it's
Read more
How is a CO₂ recovery system designed to fit different industries?
How is a CO₂ recovery system designed to fit different industries? You're under pressure to implement a CO₂ recovery solution.
Read more
How energy-efficient are today’s CO₂ recovery technologies?
How energy-efficient are today’s CO₂ recovery technologies? You want to recover CO₂, but you fear that high electricity bills will
Read more