How does a CO₂ recovery system capture and purify carbon dioxide?
•
How does a CO₂ recovery system capture and purify carbon dioxide?
Your industrial process vents a mix of gases every day. You know there is valuable CO₂ in that stream, but separating it seems like a complex and expensive chemical puzzle.
A CO₂ recovery system uses a liquid chemical solvent, typically an amine solution, to selectively absorb CO₂ from a gas stream. The system then heats this solvent to release the CO₂, which is then compressed, dried, and further polished to achieve food-grade purity (99.9%+).
I've walked through dozens of plants, from breweries to power stations, and the core challenge is always the same: how to efficiently grab just the CO₂ molecules and leave everything else behind. It's not magic, but a very precise application of chemistry and engineering. Think of it as a molecular filter that is specifically designed to catch one thing and let all other things pass through. Understanding this process, from the initial capture to the final polish, is the first step in seeing how your factory can turn a waste stream into a valuable, high-purity product.
What are the main steps in a CO₂ recovery process?
Seeing a full CO₂ recovery plant can be intimidating. With all its pipes, pumps, and vessels, the process can look like an indecipherable black box, making it hard to understand.
The process has four main steps: Absorption, where a solvent captures the CO₂. Stripping, where heat releases the CO₂. Compression, which increases the gas pressure. And finally, Purification and Liquefaction, where it's cleaned and turned into a liquid.

When I'm explaining this to a new client, I always break it down into these four distinct jobs. Each part of the system has one primary function. It's like an assembly line, but for molecules. We guide the CO₂ from a dilute, mixed gas to a concentrated, pure liquid, one step at a time. This logical flow is what makes the technology so reliable and effective. If you understand these four stages, you understand the heart of how our systems work to create value from your emissions.
The Journey of a CO₂ Molecule
Let's follow a molecule of CO₂ as it moves through one of our plants. This journey turns it from a component in a mixed gas into a marketable product.
| Stage | Purpose | Key Equipment |
|---|---|---|
| 1. Absorption | Selectively capture CO₂ from the raw gas stream. | Absorber Column |
| 2. Stripping/Regeneration | Release the captured CO₂ from the solvent using heat. | Stripper & Reboiler |
| 3. Compression | Increase the pressure of the CO₂ gas. | Multi-stage Compressor |
| 4. Purification & Liquefaction | Remove final impurities and turn the gas into a liquid. | Dryer, Filters, Condenser |
First, the raw gas enters the bottom of the absorber column and flows upward. Our special amine solvent flows down, and its chemistry allows it to grab onto the CO₂ molecules. The leftover gases, like nitrogen, exit from the top. Next, the CO₂-rich solvent goes to the stripper, where it is heated by the reboiler. This heat reverses the reaction, releasing almost pure CO₂ gas. The lean solvent is then cooled and returned to the absorber to start the cycle again. The captured CO₂ gas then goes to a large compressor. Finally, the high-pressure gas passes through dryers and carbon filters to remove trace amounts of water and other impurities before it is chilled into a high-purity liquid.
How is CO₂ separated from other gases like nitrogen or oxygen?
Your flue gas is mostly nitrogen, an inert and unreactive gas. How can a system possibly pick out the minority CO₂ molecules from this overwhelming majority of other gases?
The separation relies on chemical selectivity. The amine solvent is chemically designed to react and bond with acidic gases like CO₂, while inert, non-acidic gases like nitrogen and oxygen do not react and simply pass through the system untouched.

This is the cleverest part of the whole process. It's not a physical filter with tiny holes. It's a chemical reaction. I often explain it like having a large crowd of people (the gas stream) walk past a line of magnets (the amine solvent). If only some of the people have metal in their pockets (the CO₂ molecules), the magnets will only grab them. The rest of the people (nitrogen, oxygen) will just walk right past without being affected. Our amine solvent acts as that "chemical magnet" for CO₂. It's a targeted approach that is incredibly effective at isolating just the molecules we want to capture.
The Principle of Chemical Affinity
The success of this separation lies in the basic chemistry of the molecules involved.
- Carbon Dioxide (CO₂): In the presence of water, CO₂ is a weak acid.
- Amine Solvent: Amines are alkaline, or basic, compounds.
- Nitrogen (N₂) & Oxygen (O₂): These gases are largely inert and non-reactive under these conditions.
The fundamental principle is a simple acid-base reaction. The amine has a strong chemical affinity for CO₂ and readily forms a weak, reversible chemical bond with it inside the absorber column. Because nitrogen and oxygen are not acidic, they have no chemical affinity for the amine solvent. They do not react. Therefore, as the mixed gas bubbles up through the downward-flowing amine, only the CO₂ is captured. The rest of the gas stream, which can be over 85% nitrogen, simply continues up the column and is vented back to the atmosphere, now stripped of most of its CO₂. This high degree of selectivity is why amine scrubbing remains the industry standard for post-combustion capture.
What purification technologies are commonly used in CO₂ recovery units?
Capturing the CO₂ is only half the battle. To be useful for industries like food and beverage, the CO₂ must be exceptionally pure. Meeting these strict standards seems like a huge challenge.
After the main separation, a multi-stage purification train is used. This typically includes water separators to remove moisture, activated carbon filters to remove hydrocarbons and odor, and sometimes a catalytic converter to eliminate trace impurities like sulfur compounds.
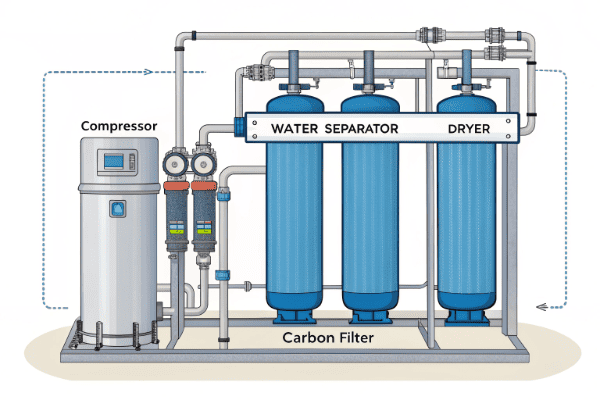
I always stress to my clients that we don't just sell a capture unit; we sell a complete purification system that delivers a specific product quality. The final purity is what determines the value of your recovered CO₂. You cannot put 99.5% pure CO₂ into a soft drink. It must be 99.9% or better, free from any compounds that could affect taste or safety. This is where the final, crucial steps of the process come into play. After the CO₂ is released from the stripper, we treat it as a product that needs to be refined to perfection.
The Path to Food-Grade Purity
Achieving this level of quality requires several dedicated purification units working in series.
- Compression and Cooling: The first step is to compress the CO₂ gas. As it's compressed and cooled between stages, a significant amount of water vapor condenses and is removed in knockout pots.
- Water Dehydration: The gas then flows through a dehydration unit, which typically uses desiccant materials like molecular sieves to absorb any remaining water vapor. This is critical to prevent ice and corrosion issues later on.
- Activated Carbon Filtration: Next, the dry CO₂ passes through large vessels filled with activated carbon. The massive surface area of the carbon adsorbs volatile organic compounds (VOCs), sulfur compounds, and other trace contaminants that could cause off-flavors or odors.
Finally, the cold, high-pressure, ultra-pure CO₂ gas is sent to a condenser, where it is chilled until it becomes a liquid, ready for storage in insulated tanks.
Conclusion
CO₂ recovery works by using a selective solvent to capture the CO₂, then releasing it with heat. A final purification train ensures the end product meets the high-purity standards required for industrial use.
You may also be interested in:

Why is CO₂ recovery technology gaining popularity worldwide?
Why is CO₂ recovery technology gaining popularity worldwide? You see headlines about carbon capture everywhere. But you wonder if it's
Read more
How is a CO₂ recovery system designed to fit different industries?
How is a CO₂ recovery system designed to fit different industries? You're under pressure to implement a CO₂ recovery solution.
Read more
How energy-efficient are today’s CO₂ recovery technologies?
How energy-efficient are today’s CO₂ recovery technologies? You want to recover CO₂, but you fear that high electricity bills will
Read more